Guaifenesin NR: Package Insert / Prescribing Info
Package insert / product label
Generic name: guaifenesin
Dosage form: syrup
Drug class: Expectorants
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Guaifenesin NR Description
Guaifenesin NR syrup is an expectorant available in liquid form for oral administration.Each 5 mL (1 teaspoonful) contains: Guaifenesin 100 mg.
INACTIVE INGREDIENTS: Caramel, Citric Acid, Flavor, Glycerin, Propylene Glycol, Saccharin Sodium, Sodium Benzoate, Sorbitol Solution, Water. It may contain Sodium Citrate.
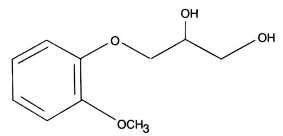
Guaifenesin (glyceryl guaiacolate) has the chemical name 3-(2-methoxyphenoxy)-1,2-propanediol. Its molecular formula is C10H14O4, with a molecular weight of 198.21. It is a white, colorless crystalline substance with a slightly bitter aromatic taste. One gram dissolves in 20 mL water at 25°C; freely soluble in ethanol. Guaifenesin is readily absorbed from the gastro-intestinal tract and is rapidly metabolized and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite is beta-(2-methoxyphenoxy) lactic acid.
Guaifenesin NR - Clinical Pharmacology
Guaifenesin is an expectorant, the action of which promotes or facilitates the removal of secretions from the respiratory tract. By increasing sputum volume and making sputum less viscous, guaifenesin facilitates expectoration of retained secretions.
Indications and Usage for Guaifenesin NR
Helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus, drain bronchial tubes, and make coughs more productive. Helps loosen phlegm and thin bronchial secretions in patients with stable chronic bronchitis.
Contraindications
This product is contraindicated in patients hypersensitive to any of the ingredients.
Precautions
Carcinogenesis, Mutagenesis, Impairment of Fertility: Animal studies to assess the long-term carcinogenic and mutagenic potential or the effect on fertility in animals or humans have not been performed.
Pregnancy
Teratogenic Effects-Pregnancy Category C: Animal reproduction studies have not been conducted. Safe use in pregnancy has not been established relative to possible adverse effects on fetal development. Therefore, this product should not be used in pregnant patients, unless in the judgment of the physician, the potential benefits outweigh possible hazards.
Nursing Mothers: It is not known whether guaifenesin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when these products are administered to a nursing woman and a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Laboratory Test Interactions: Guaifenesin or its metabolites may cause color interference with the VMA (vanilylmandelic acid) test for catechols. It may also falsely elevate the level of urinary 5-HIAA (5-hydroxyindoleacetic acid) in certain serotonin metabolite chemical tests because of color interference.
Adverse Reactions/Side Effects
Guaifenesin is well tolerated and has a wide margin of safety. Side effects have been generally mild and infrequent. Nausea and vomiting are the side effects that occur most commonly. Dizziness, headache and rash (including urticaria) have been reported rarely.
Overdosage
The acute toxicity of guaifenesin is low and overdosage is unlikely to produce serious toxic effects. In laboratory animals no toxicity resulted when guaifenesin was administered by stomach tube in doses up to 5 grams/kg.
In massive over dosage the stomach should be emptied (emesis and/or gastric lavage) and further absorption prevented. Treatment is symptomatic and supportive.
Guaifenesin NR Dosage and Administration
Adults and children 12 years of age and older: Two to four teaspoonfuls (200 mg to 400 mg) every four hours, not to exceed 2400 mg (24 teaspoonfuls) in 24 hours.
Children 6 years to under 12 years of age: One to two teaspoonfuls (100 mg to 200 mg) every four hours, not to exceed 1200 mg (12 teaspoonfuls) in 24 hours.
Children 2 to under 6 years of age: 1/2 to 1 teaspoonful (50 mg to 100 mg) every four hours, not to exceed 600 mg (6 teaspoonfuls) in 24 hours.
Children 6 months to under 2 years of age: A common dosage is 1/4 to 1/2 teaspoonful (25 mg to 50 mg) every 4 hours, not to exceed 300 mg (3 teaspoonfuls) in 24 hours. Individualized dosage should be determined by evaluation of patient.
How is Guaifenesin NR supplied
Guaifenesin NR Liquid is available in one pint (473 mL), and one gallon (3785 mL).
Store at controlled room temperature 15°-30°C (59°-86°F).
PROTECT FROM LIGHT. KEEP BOTTLE TIGHTLY CLOSED.
Dispense in a tight container as defined in the USP.
Manufactured by:
Silarx Pharmaceuticals, Inc.
Spring Valley, NY 10977
| GUAIFENESIN NR LIQUID
guaifenesin nr liquid liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Silarx Pharmaceuticals, Inc (161630033) |
Frequently asked questions
- Does Mucinex help you get pregnant?
- Does Mucinex help with Covid?
- What is the dose for an adult for Robitussin DM? How many times a day?
More about guaifenesin
- Compare alternatives
- Pricing & coupons
- Reviews (300)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: expectorants
- Breastfeeding
Patient resources
Professional resources
Other brands
Mucinex, Mucinex Maximum Strength


